Sub-retinal injection gene therapy revs up anti-VEGF drugs
A special sub-retinal injection to help boost the therapeutic powers of anti-VEGF medications is being studied. So far, study patients […]

Sub-retinal injection gene therapy revs up anti-VEGF drugs
A special sub-retinal injection to help boost the therapeutic powers of anti-VEGF medications is being studied. So far, study patients […]
A special sub-retinal injection to help boost the therapeutic powers of anti-VEGF medications is being studied.
So far, study patients have enjoyed much more success than they’ve had in their past treatments for wet age-related macular degeneration (AMD).
Avalanche Biotech is a biotechnology company that develops products for delivery of therapeutic proteins to the eye to treat wet age-related macular degeneration (AMD). Avalanche co-founder, Thomas Chalberg, PhD, reported that Phase 1 of a randomized clinical trial has been completed. Dr. Chalberg reports that patients treated with their sub-retinal injection gained between 9 and 12 letters on the eye chart after 12 months.
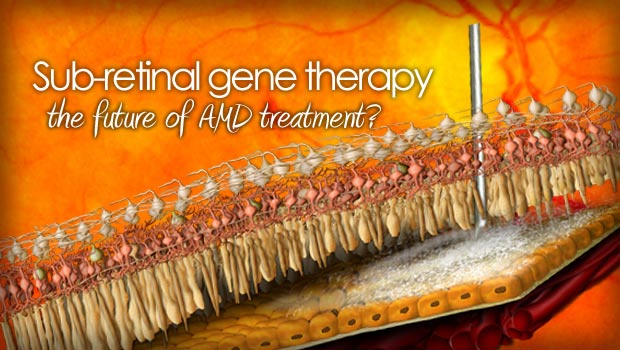
 How the subretinal injection works
How the subretinal injection works
AVA-101 (Ocular BioFactory™, Avalanche Biotechnologies) is a therapeutic DNA strand that is packaged inside an Adeno-Associated Virus (AAV). AAV is a very safe virus that is used in many medical therapies for diseases like ALS, Alzheimer’s Disease and Parkinson’s disease. In this case, the viral genes are removed from the AAV virus and replaced with therapeutic proteins known to slow the effects of AMD.
In the study, one hundred microliters of AVA-101 was injected under the retina in participating patients. The virus unloads its therapeutic DNA payload into the affected areas throughout the lower layers of the retina. This creates a “Biofactory™” of proteins that boost the body’s use of anti-VEGF drugs.
Why is it injected under the retina?
AVA-101, when injected into the sub-retinal space, instead of into the vitreous (like the anti-VEGF medications), appeared in the study to create better stimulation of anti-VEGF than if injected into the vitreous. Sub-retinal injection caused no adverse reactions, retinal detachments or tears. It appeared to be a very safe mode of delivery.
How the study was done
Patients were treated then followed in the study for 12 months. Individuals were specifically chosen if they had an extensive history of anti-VEGF treatments. The average number of prior treatments per patient in the study was 18. In other words, these subjects were experienced intra-vitreal, anti-VEGF therapy patients.
At the beginning of the 12-month study, some eyes were given the sub-retinal injection of AVA-101. During the following 6-8 weeks, the eyes were given two separate treatments of a popular anti-VEGF medication, Ranibizumab (Lucentis). The study was designed to allow for anti-VEGF injections to be given on an as-needed “rescue” basis only, after the first 8 weeks. If the patient had a significant decrease in vision, or a diagnostic Optical Coherence Tomography (OCT) or fluorescein angiogram demonstrated worsening of the wet AMD, the patient was given a rescue treatment of Lucentis.
Study results
The control group (the patients who did NOT receive the AVA-101) ended up requiring an average of 3 Lucentis injections during the 12-month study period due to worsening of the AMD disease. The treatment group (the patients who DID receive the initial AVA-101 sub-retinal injection) required an average of 0.3 Lucentis injections over the 12-month period.
Patients getting the AVA-101 therapy enjoyed a gain of between 9 and 12 letters (2-4 lines) on the eye chart by the end of the study. No drug-related complications were seen and there were no instances of retinal detachment or tears.
These results are significant. At the moment of this writing, Phase 2A of the study has forty patients enrolled. We will post information here at www.MaculaCenter.com regarding the results of this second phase of the study.