Anti-VEGF excels at treating macular edema
A study showed that Eylea, a popular macular degeneration drug, had better success in treating macular edema caused by branch […]


Anti-VEGF excels at treating macular edema
A study showed that Eylea, a popular macular degeneration drug, had better success in treating macular edema caused by branch […]
A study showed that Eylea, a popular macular degeneration drug, had better success in treating macular edema caused by branch retinal vein occlusion than traditional laser treatment
The study recently concluded that treating macular edema that is caused by branch retinal vein occlusion (BRVO), with aflibercept (Eylea) injections, was more successful than retinal laser photocoagulation. Laser has been the standard, “go-to” treatment for BRVO for decades. Eylea is not presently (as of July, 2014) FDA approved for treating macular edema in BRVO.
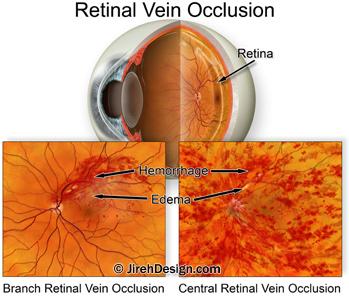
Branch retinal vein occlusion (BRVO) is an eye condition that happens when one of the smaller veins on the retina, at the back-inner wall of the eye, becomes blocked by a blood clot, or “pinched off”, causing some of the blood flowing through the vein to bleed out of the vein wall. BRVO can also cause leaking of the weakened retinal blood vessels. The result is macular edema and subsequent blurry vision.
A central retinal vein occlusion (CRVO) happens when the large retinal vein, responsible for transporting blood out of the eye, becomes blocked. CRVO symptoms usually involve extreme loss of vision. CRVO is already an approved condition for using Eylea.
Anti-VEGF drugs work by slowing or stopping abnormal blood vessel leaking caused by various eye diseases. These anti-VEGF drugs are injected intravitreally (into the vitreous gel filling the inside of the eyeball), typically on a regular basis, and have a powerful anti-inflammatory response.
Ophthalmic use of Anti-VEGF drugs was originally FDA-approved for the treatment of neovascular (wet) AMD in 2005. However, recent studies have demonstrated that this family of drugs can be used safely and effectively for a number of retina conditions that involve macular edema as a secondary complication – diabetic retinopathy, for one.
It is important to note that Genentech’s anti-VEGF drug, Lucentis, is presently approved for treatment of macular edema secondary to retinal vein occlusion, both BRVO and CRVO. Though it works somewhat differently on a molecular level, Lucentis is also in the anti-VEGF drug family with Eylea.
Although retinal laser photocoagulation is still indicated and safe for treatment of BRVO, the VIBRANT trial findings suggest a more successful, less retina-damaging treatment for macular edema caused by BRVO. Laser will remain as an important adjunct treatment in certain cases.
These findings could lead to the Federal Drug Administration approving Eylea for treatment of macular edema, secondary to retinal vein occlusion.
Bookmark www.MaculaCenter.com and visit frequently for updates on BRVO research and treatments.