Artificial retina in phase 2
Clinical trials have begun on a second-generation artificial retina implant California — The FDA has recently approved new clinical trials […]


Artificial retina in phase 2
Clinical trials have begun on a second-generation artificial retina implant California — The FDA has recently approved new clinical trials […]
Clinical trials have begun on a second-generation artificial retina implant
California — The FDA has recently approved new clinical trials to test the latest artificial retina implant model — the Argus II.
Researchers at the Doheny Eye Institute at the University of Southern California Medical Center have begun enrolling study patients with retinitis pigmentosa (RP) — a blinding retinal disease.
The Argus I artificial retina model has already undergone human implant tests that indicated the safety and long-term reliability of the implants.
During the Argus I’s testing phase, six RP patients received the retina prosthesis. Each of these previously blind patients have been able to distinguish and identify objects and motion.
The new, smaller Argus II is believed to be safer and more effective.
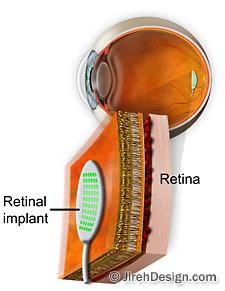
How it works
Vision begins when light enters the eye through the cornea, moves through the lens and vitreous and strikes the retina, the inner-most lining of the eyeball. The retina, a light-sensitive layer of tissue, sends visual messages through the optic nerve to the brain, where the visual impulses are interpreted as an image. Watch the “How vision works” animation
The Argus I artificial retina model has already undergone human implant tests
Retinal diseases like, age-related macular degeneration and retinitis pigmentosa decrease vision by destroying the light-sensitive photoreceptor cells in the retina.
With the artificial retina device, a miniature camera mounted in eyeglasses captures images and wirelessly sends the information to a microprocessor (worn on a belt) that converts the data to an electronic signal and transmits it to a receiver on the eye. The receiver sends the signals through a tiny cable to the microelectrode array, stimulating it to emit pulses.
The artificial retina device thus bypasses defunct photoreceptor cells and transmits electrical signals directly to the retina’s remaining viable cells. The pulses travel to the optic nerve and, ultimately, to the brain, which perceives patterns of light and dark spots corresponding to the electrodes stimulated. Patients learn to interpret these visual patterns.
Clinical trials on a third model are projected to begin in 2011.
It is important to note that, as of the March ’08, all artificial retina devices are still experimental and are not yet commercially available.
*Dr. Deupree and The Macula Center do not perform artificial retina implants.
Some information provided by U.S. Dept. of Energy: ArtificialRetina.energy.gov